Binding partners for FGF1 in nucleocytoplasmic translocation
FGF-1 possess broad mitogenic and cell survival activities, and is involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. Very important part in FGF-1 functioning is connected with its translocation within a cell – from cytoplasm to nucleus. There are several proteins discovered to be binding partners for FGF-1, helping it in its activities. It has been shown that FGF-1 for sure interacts specifically with intracellular proteins, such as FIBP, p34 and CK2 but it has been also initially described that there are new potential binding partners: nucleolin, nucleophosmin and major vault protein (MVP).
Major vault protein, nucleolin and nucleophosmin are potential binding partners for FGF-1. It was shown by means of mass spectrometry that these proteins, among some others, were present after performing fishing experiments using streptavidin tag attached to FGF-1 and streptavidin dynabeads. However, it is not clear if these three proteins interact with FGF-1 directly or if they just exist in a complex of a few proteins interacting with FGF-1. As MVP, nucleolin and nucleophosmin are very interesting intracelular proteins we decided to study these probable interactions as well as the colocalization in the cell and the fates of these partner proteins.
Major vault protein is a main protein of vault particles which are present in large numbers (10.000 – 1.000.000 vaults per cell) in the cytoplasm of most eukaryotic cells. MVP is thought to be present in 96 copies per vault, based on the observed symmetry of the particle and the fact that MVP accounts for 75% of the total protein mass of the particle.
MVP takes part in transport mechanisms, signal transmission and immune responses and is overexpressed in multi-drug resistance cells.
Nucleolin is DNA and RNA binding protein involved in regulation of gene transcription, chromatin remodeling, RNA metabolism and ribosomal RNA synthesis. Nucleolin contains several functional domains that mediate its functions. The N-terminal part contains multiple phosphorylation sites and is rich in acidic amino acids. The central part of nucleolin includes four RNA binding domains (RBD) and the C-terminal part contains glycine and arginine rich domain (termed RGG or GAR domain).
Nucleophosmin is a protein that is involved in regulation of cell growth, proliferation, apoptotic response to stress, oncogenic stimuli (such as DNA damage and hypoxia), and it can modulate the activity and stability of crucial tumour suppressor proteins such as p53.
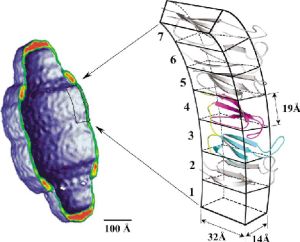
Fig. Model of vaults. MVP structural repeats 2, 5, 6, and 7 (gray) were modeled based on the 3-4 domain structure (color) and placed to form a continuous wall comprising the widest part of the vault particle. (Kozlov G. et al. (2006). Solution Structure of a Two-repeat Fragment of Major Vault Protein. J. Mol. Biol. 356, 444-452).




